What exactly is this "scientific breakthrough" or "progress" announced on June 17th?
"According to our own internal evaluation, this is a progress," Fulong Liao, a member of Tu's team and a researcher at the Chinese academy of traditional Chinese medicine, was quoted as saying by sci-tech daily. This view was also recognized by other team members, such as researcher Jigang Wang.

An article published in the New England journal of medicine is not a research paper per se, but an opinion piece. The focus of this paper is to present the authors' views on changing artemisinin resistance and the reasons for the change in treatment regimens, without directly supporting the data on the effectiveness of improved treatment regimens. So it is not appropriate to call an opinion piece (as opposed to a research paper) proposing clinical treatment a "breakthrough".

So what is this development?
Artemisinin, an effective and cheap anti-malaria drug, "has seen a 50 per cent drop in malaria deaths and a 40 per cent drop in infections in the last 10 years, partly because of Tu's discovery". "Said Yan·Andersson, a member of the Nobel committee.
But little is known about the challenges artemisinin has faced in treating malaria in recent years. Areas including Cambodia, myanmar, Thailand, Laos and China are already showing signs of "drug resistance" -- artemisinin cures malaria more slowly and, in some cases, is no longer effective.
Progress is likely to lead to a solution to drug resistance.
Break the tradition of "3-day therapy"
Artemisia annua has two main characteristics.
One is that artemisinin works by alkylation of proteins from both heme and plasmodium. The alkylated heme is not broken down by the malaria parasite, and the alkylated plasmodium protein itself is a disservice - which means that artemisinin hits the malaria parasite in so many different ways that it is difficult for them to gain resistance through small mutations.
Second, artemisinin is initially "dormant" and needs to be activated by iron and heme, the hemoglobin that malaria parasites break down, to be effective. But malaria parasites have many life cycles and sometimes go dormant. When they do, they don't break down too much hemoglobin, and they don't have enough iron and haemoglobin to activate artemisinin, like "meeting the right person at the wrong time."
The white powder is artemisinin:

Will it be the next step?
Another potential application of artemisinin is to treat systemic lupus erythematosus.
Systemic lupus erythematosus (SLE) has been a staple of medical dramas because it's so tricky:
· autoimmune diseases with unknown etiology;
· it can harm almost all organs in the body, but the condition of patients and patients can be very different;
· some patients' kidneys, blood system, central nervous system and other key parts will be seriously damaged, such as lupus nephritis;
· there are no drugs that can be cured, and the treatment goal is to ensure long-term survival, prevent organ damage and the ideal health-related quality of life by controlling disease activity, reducing complications and drug toxicity;
· in most developed countries, more than 95% of patients survive at least 10 years after diagnosis;
· there are about 1 million patients in China, most of whom are young women of childbearing age.
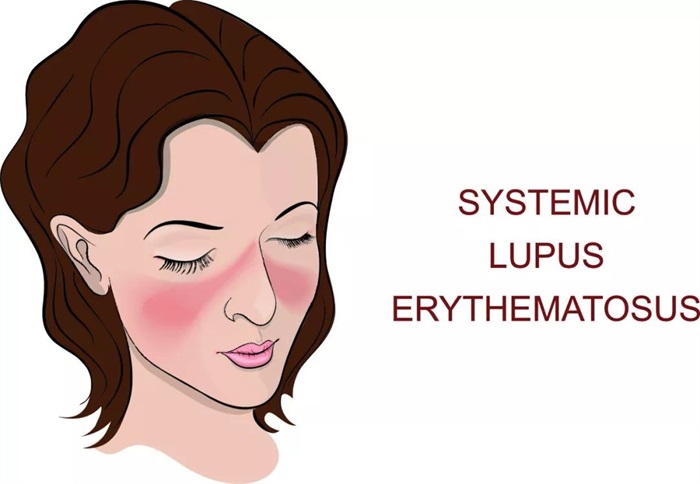
Symmetrical butterfly erythema is one of the typical symptoms of lupus erythematosus
Drugs used to treat SLE include hormones, immunosuppressants, monoclonal antibodies, etc. The classic antimalarial, hydroxychloroquine, is a second-line treatment for systemic lupus erythematosus. In fact, antimalarials have been used in the treatment of lupus for a long time: quinine was reported to be effective in treating lupus rashes in 1894.
Attempts to treat lupus with artemisinin also began very early. In 1979, a paper suggested that artemisinin could treat discoid lupus erythematosus, a relatively mild form of the disease. In the same year, artemisinin was found to have immunosuppressive effects in the screening of Chinese herbal immune promoters and immunosuppressants. After dihydroartemisinin was approved as a class of new drugs in 1992, tu youyou began to focus on artemisinin's treatment of autoimmune diseases.
What is the effect of artemisinin on SLE? This needs to be confirmed by larger, more orderly clinical trials.
The most optimistic estimate is 2026.
It is important to note, however, that artemisinin is not a once-and-for-all magic pill for SLE. Like other drugs, it requires long-term use. There are reasonable expectations for this potential new drug.
We believe that today's "big breakthroughs" will really come to us in the near future.
Society in progress, TUMTEC R&D department will continue to develop new technology, continue to break through themselves, research and development of more advanced splicing machine and cleaver.

Links
Magic Lamp